Results for 'Life Reset Labs'

New Book Unites Oncology’s Brightest Minds To Innovate Cancer Cures
Sep 8th • 5 mins read


Combatting the “Summer Slow Down" – MSL Job Search Tips for Slower Months
Jun 22nd • 2 mins read




Assessment of Coverage in England of Cancer Drugs Qualifying for US Food and Drug Administration Accelerated Approval
Feb 21st • 10 mins read

Assessment of Food and Drug Administration- and European Medicines Agency-Approved Systemic Oncology Therapies and Clinically Meaningful Improvements in Quality of Life: A Systematic Review
Feb 10th • 4 mins read
Accelerated drug approvals in oncology: Pros and cons
Sep 13th • 4 mins read
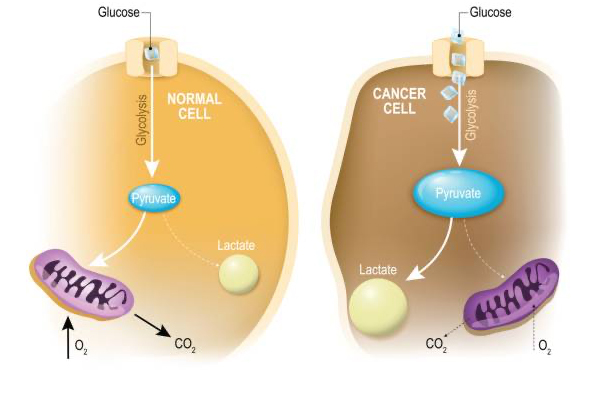
“Oncometabolism: The switchboard of cancer: An editorial”
Jan 31st • 1 min read
Clinical benefit of immune checkpoint inhibitors approved by US Food and Drug Administration
Aug 30th • 16 mins read

Clinical benefit and cost of breakthrough cancer drugs approved by the US Food and Drug Administration
Jul 21st • 12 mins read

Assessment of Clinical Trials Supporting US Food and Drug Administration Approval of Novel Therapeutic Agents, 1995-2017
Apr 20th • 20 mins read

Proportion of Patients in Phase I Oncology Trials Receiving Treatments That Are Ultimately Approved
Mar 31st • 14 mins read

Publicly accessible evidence of health-related quality of life benefits associated with cancer drugs approved by the European Medicines Agency between 2009 and 2015
Feb 22nd • 12 mins read
EHA evaluation of the ESMO-Magnitude of Clinical Benefit Scale version 1.1 (ESMO-MCBS v1.1) for hematological malignancies
Jan 19th • 20 mins read
Pivotal Considerations for Optimal Deployment of Healthy Volunteers in Oncology Drug Development
Oct 30th • 20 mins read

Cost per Event Averted in Cancer Trials in the Adjuvant Setting From 2018 to 2022
Jun 9th • 30 mins read

Clinical benefit of cancer drugs approved in Switzerland 2010–2019
Jun 9th • 35 mins read

A Comprehensive Comparison of Additional Benefit Assessment Methods Applied by Institute for Quality and Efficiency in Health Care and European Society for Medical Oncology for Time-to-Event Endpoints After Significant Phase III Trials—A Simulation Study
Jun 27th • 30 mins read
